Sample Device History Record
Sample device history record submerged oxygen salt water sample device sample device history record sample device for liquids sample device agreement soil sample device free sample devices sample devices
Sample Device History Record. Every item on this page was chosen by a town & country editor. The device history record (dhr) is outlined in the us fda quality system requirements, part 820, section 184.
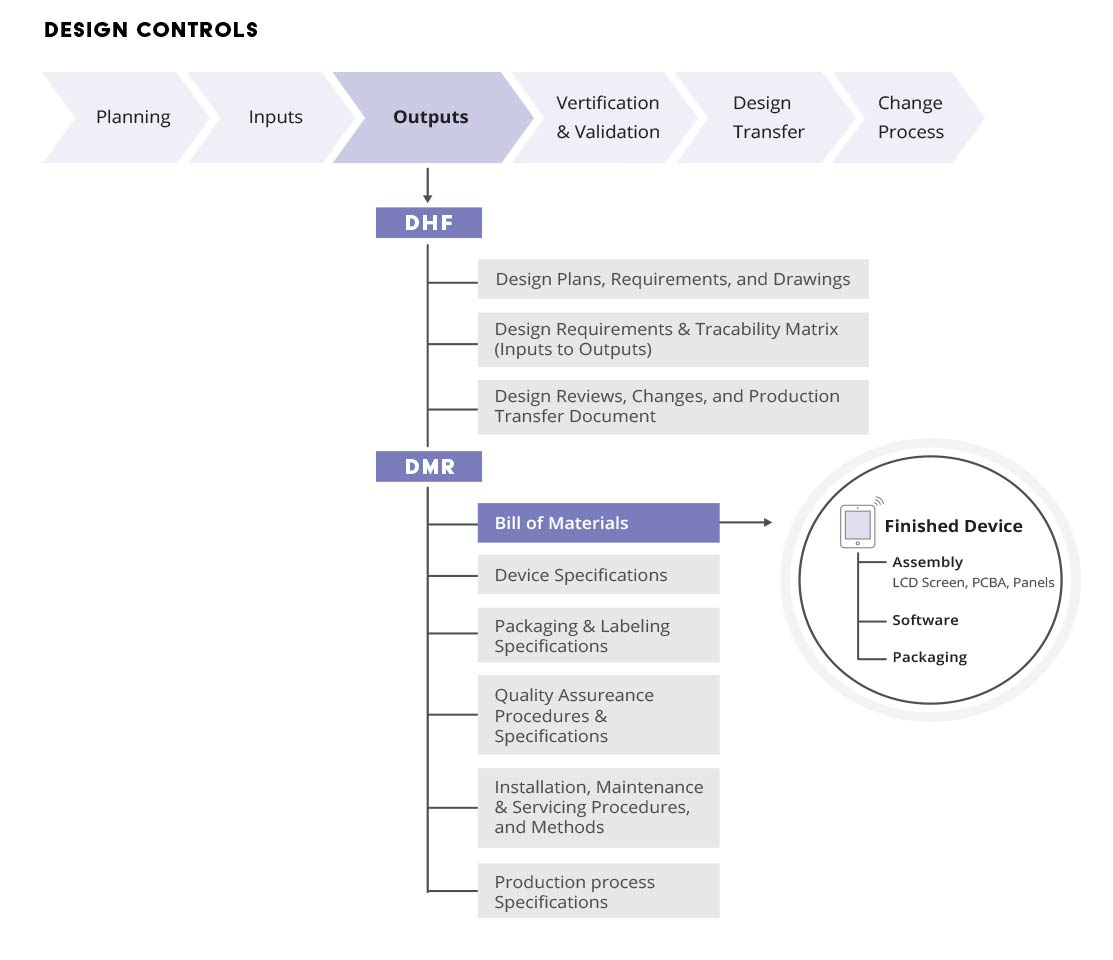
This specification, of course, may be the same as .
This specification, of course, may be the same as . The entries into this document must include acceptance records . You have, without a doubt, heard a motown hit in your lifetime. We may earn commission on some of the items you choose to buy.
